
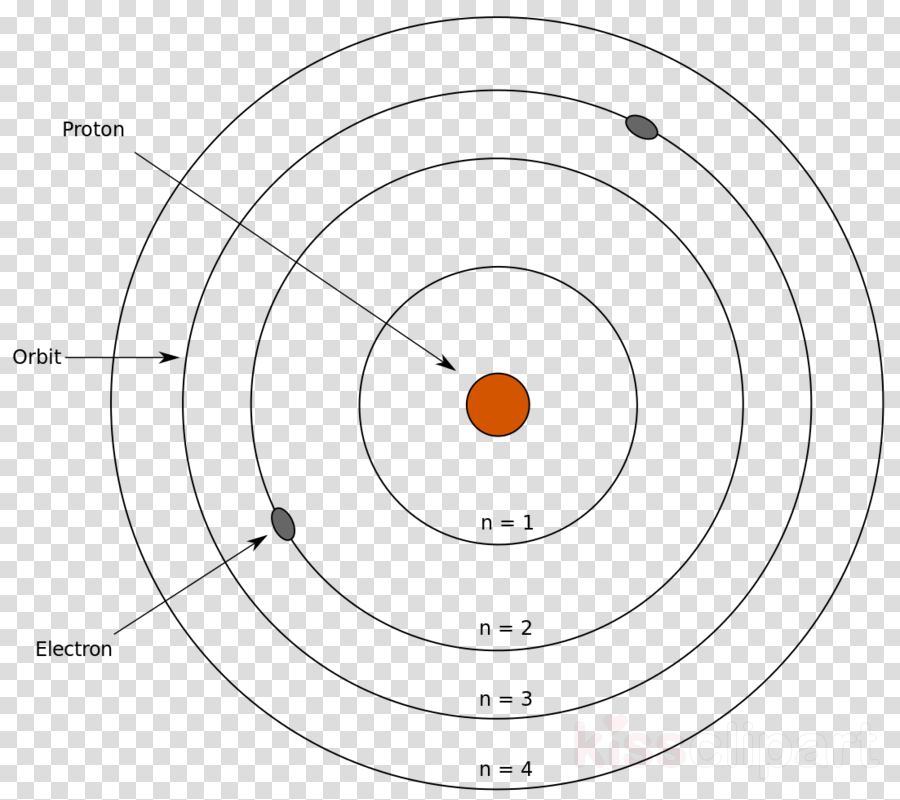
Negatively charged electron revolves about the nucleus in a circular orbit, the centripetal force required for revolution is provided by the electrostatic force of attraction between the nucleus and electrons. The first postulate states that every atom has a positively charged central core called the nucleus in which the entire mass of an atom is concentrated. There are three Bohr’s Postulates in Neil Bohr Model, each of these are described in detail below: First Postulate There are three Bohr’s postulates of atomic models, we will talk about these in detail. To overcome this, Neil Bohr combined classical ideas with the quantum concepts of Planck to give something, which is known as the Neil Bohr atomic model of Hydrogen.
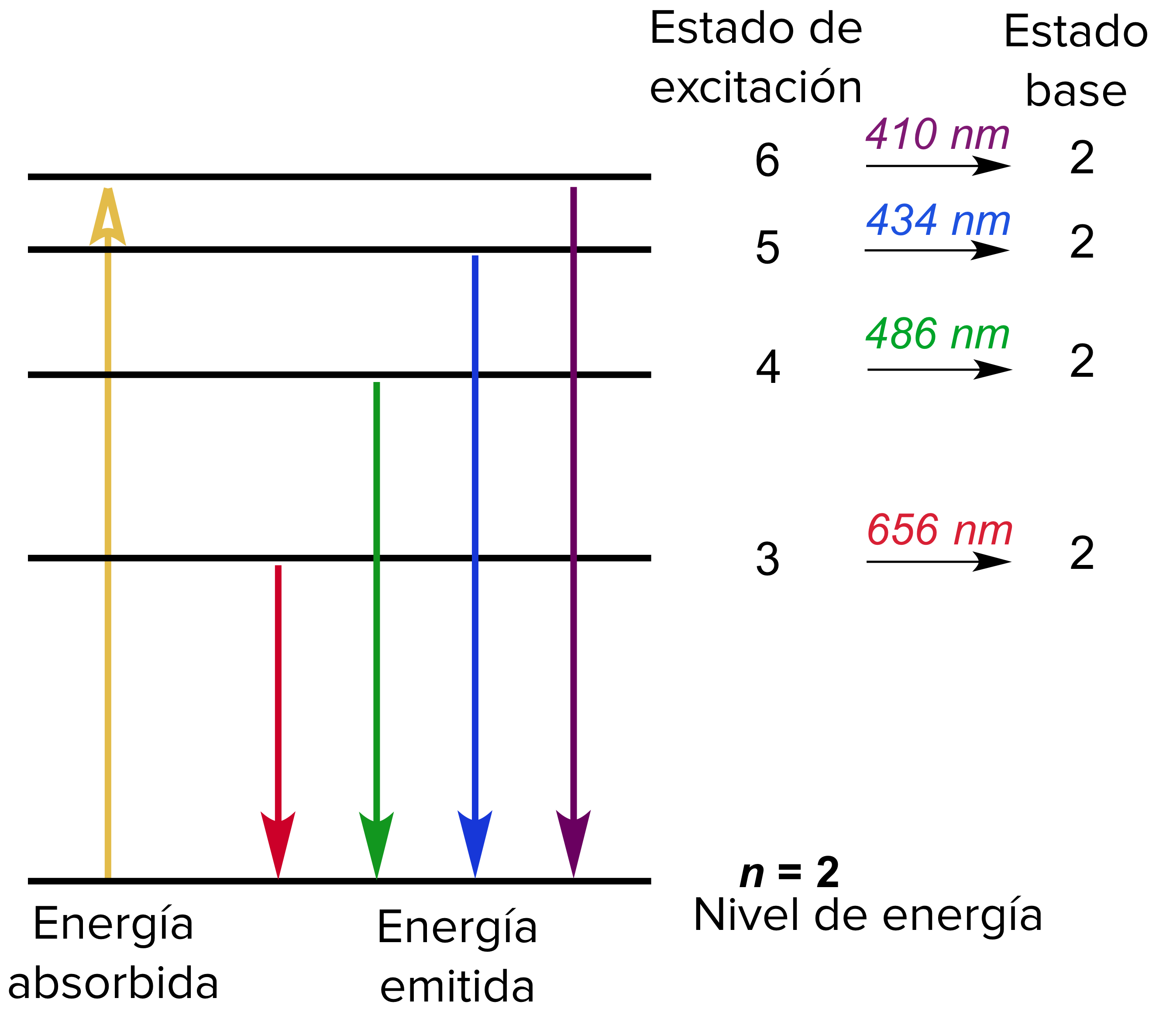
Viewing the demerits of the Rutherford model, Neil Bohr concluded that classical mechanics and electromagnetism cannot be applied to the processes on the atomic scale. The transfer of electrons is possible by emission and absorption of energy. The orbits are symbolized with the letter ‘n’, where the value of n is an integer. Bohr's Model explained how electrons travel in different circular orbits around the nucleus.


 0 kommentar(er)
0 kommentar(er)
